
In the case of niobium, 93 minus 41 is 52, which means that a niobium atom has 52 neutrons. Finally, subtract the number of protons from the rounded up atomic weight to find the number of neutrons in the atom.
/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
Niobium has an atomic weight of 92.906, so you would round it up to 93. Round up the atomic weight to the nearest whole number. Express the changes in the atomic number and mass number of a radioactive nuclei when an alpha, beta, or gamma particle is emitted. Together, the number of protons and the number of neutrons determine an element's mass number: mass number protons + neutrons.
#Equation for atomic mass number series#
Next, find the atomic weight of the element, which is usually underneath the element symbol. 2 Solution Decay Series Summary Vocabulary Learning Objectives Compare qualitatively the ionizing and penetration power of alpha particles ( ), beta particles ( ), and gamma rays ( ). Forms of the same atom that differ only in their number of neutrons are called isotopes. For instance, the atomic number of niobium (Nb) is 41, meaning that a niobium atom has 41 protons. 2 It is also the number of electrons because, elements have no overall charge as the positive protons cancel out the negative electrons. The sum of the mass number and the atomic number for an atom (A-Z) corresponds to the total number of subatomic particles present in the atom. This number represents the number of protons in the atom. 1 Atomic number is the number of protons so the answer is 10. Together, the number of protons and the number of neutrons determine an element’s mass number: mass number protons + neutrons. It’s usually located somewhere above the element symbol. Contrast this with the atomic number, which is simply the number of protons. Mass number is often denoted using a capital letter A. In other words, it is the sum of the number of nucleons in an atom. Then, find the atomic number for the element. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. of protons present in the nucleus of an atom.Atomic MassThe sum of the Masses of protons, neutrons. So, we can say that the atomic mass or mass number is equal to the total number of nucleons present in the atom of an element. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less.
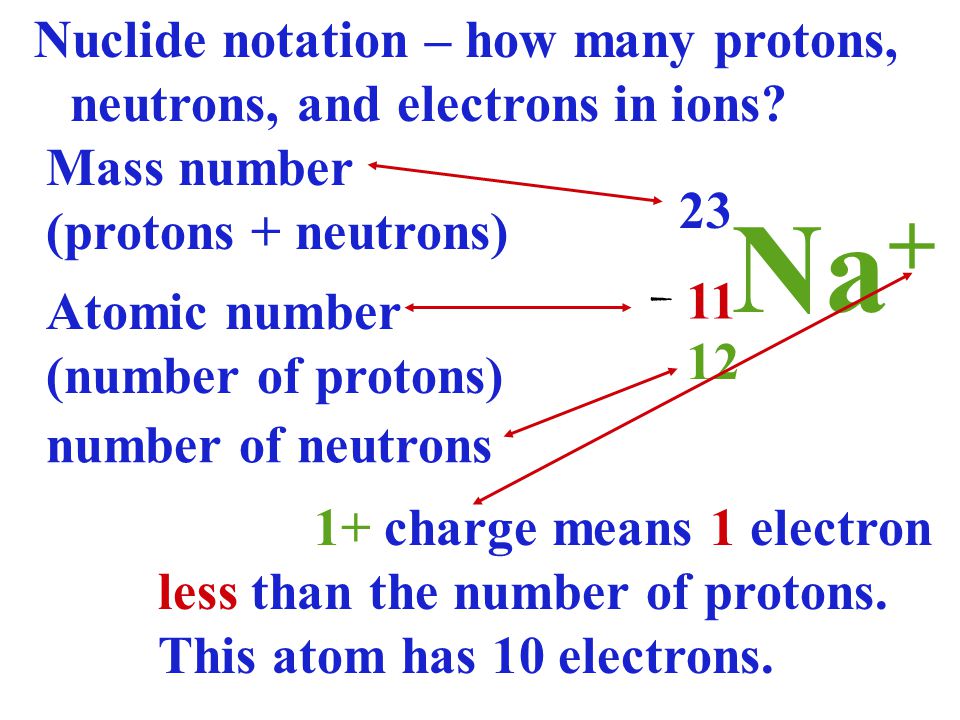
To know the atomic mass of element you may visit Atomic mass. Thus Atomic mass number of protons + number of neutrons.
First, locate the elemental symbol for your atom on the periodic table. The formula for Atomic number is-Atomic number no. During -decay, an atomic nucleus emits an alpha particle. Atomic mass is equal to the sum of protons and neutrons present in the nucleus of the atom.
#Equation for atomic mass number how to#
The sample problem below demonstrates how to calculate the atomic mass of chlorine.To find the number of neutrons in an atom, you just need a periodic table that lists the atomic number as well as the atomic weight of each element. The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. N is a constant called Avogadros number and its equal to 6.0221023 atoms/mole. Why? We need to take into account the percent natural abundance of each isotope, in order to calculate the weighted average. of atoms N (density) volume / (Molecular Weight).
\nonumber \]Ĭlearly the actual average atomic mass from the last column of the table is significantly lower.


 0 kommentar(er)
0 kommentar(er)
